Processing of grapes into raisins
At the ripening stage, grapes are harvested and transported to consumption or processing.
Ripening is characterized by the formation of a waxy layer on berry skin, softening and
development of the specific grape variety color as well as changing of taste from sour to
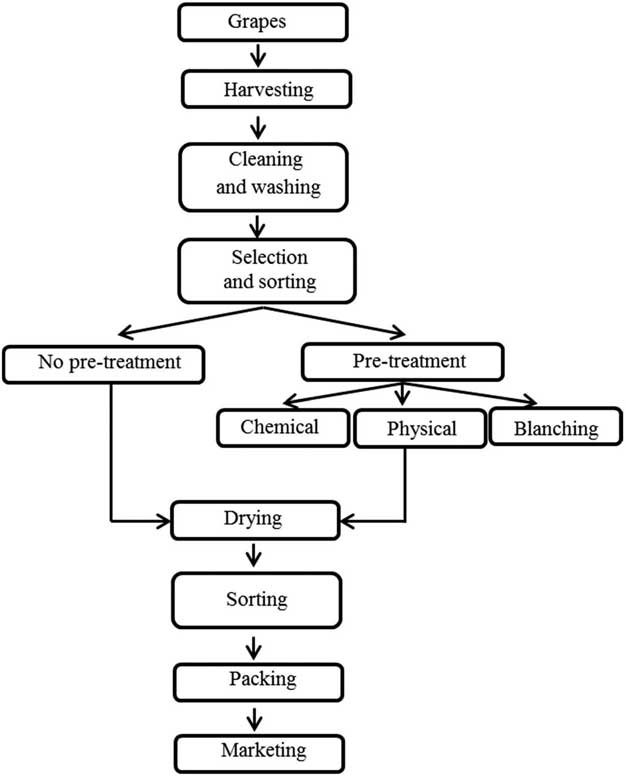
sweetness. In the case of drying, the berries are first cleaned, washed, selected and
sorted, and then subjected or not to a pretreatment that accelerates the dehydration
process. After drying, the resulting raisins are sorted once again before being packaged
and marked (Following figure: Diagram of grapes drying).
Pretreatments of grapes
As far as is currently known, there are two types of pretreatments that have been
frequently applied in raisins processing: chemical and physical treatments. Both of
these pretreatments have been the subject of numerous investigations and have as main
objectives the modification of the grape skin permeability, enhancement of moisture rates,
swiftness of the drying process as well as improvement of raisins’ quality.
Chemical pretreatments
Chemical pretreatments is used to accelerate raisin drying which involve treatment of grapes with sulfur dioxide (SO2) or dipping berries into certain chemical solutions. These include sodium carbonate (Na2CO3), potassium carbonate (K2CO3), sodium hydroxide (NaOH) and oil emulsion (e.g. ethyl oleate and olive oil) at different concentrations and times.
It is worth noting that in the grape industry, the sulfiting technique (i.e. gas SO2
fumigation or immersion in SO2 solutions) was by far the commonly-used method for
bleaching grapes during storage and drying since sulfur dioxide treatment was known to
ameliorate food color development and conserve their quality. However, excessive SO2
usage may alter the quality of the processed raisins and could cause potential environmental
problems such as air pollution or certain health concerns about reactions
associated with some types of asthma. .
Ref: FOOD REVIEWS INTERNATIONAL 3, R. Khiari ET AL.
Drying Emulsion Cold Dip
As said one of the methods of chemical pretreatment is dipping grapes into certain chemical solutions. Generally fruit dipping mixtures were developed in ancient times in the Mediterranean area and Asia Minor. Initially, they were formulated using olive oil and wood ash. In modern times, wood ash was replaced with food-grade potassium carbonate (K2CO3), and the olive oil by specially formulated dipping oils. Today, most commercial cold dips utilize a combination of potassium carbonate and ethyl esters of fatty acids (commonly referred to as ethyl oleate) as active constituents in unheated water—hence the term cold dip. This treatment increases the rate of water loss twofold to threefold, an important factor in countries where drying conditions are very unpredictable. The shortened drying time is also important in achieving the desired light-gold fruit in ‘Sultana’ production areas such as Australia. Some countries such as Turkey and Greece still use olive oil extensively because it is relatively inexpensive and plentiful. The relatively high proportion of oleic acid in olive oil accounts for the similarities in activity of traditional and newer oil derivatives.
Drying Emulsion Use and Research in Australia
Much of what we know about drying aids comes from Australian experience and research. CSIRO (Commonwealth Scientific and Industrial Research Organization) researchers have evaluated many combinations of oils or fatty acids and alkaline salts. Overall, they have found that both constituents produce a synergistic reaction to modify the berry cuticle—a reaction that cannot be achieved fully with either one alone. While the exact role of the constituents of the emulsion are not fully understood, present knowledge indicates that the fatty acids mainly modify the outer wax layer while the potash (K2CO3) neutralizes free acids and their electrical charges in the cuticular membrane. The fatty acids change the primary structure and arrangement of the wax platelets and reduce their surface tension. Upon penetration, they interact with the soluble waxes and establish a hydrophilic (water-conducting) link between the berry surface and the watercontaining parenchyma cells. They also cause the waxy layers to swell, pushing them apart. This creates a water continuum through the cuticular membrane—a water path from the saturated interior to the berry surface—thus facilitating the flow and transpiration of water. K2CO3 neutralizes any free acids present in or on the skin, including those of the wax, converting them
into their potassium salt. Absorption of water would also be aided by the neutralization of fixed charges with K+.CO3 also stabilizes the emulsion by increasing pH.
The relative effectiveness of the emulsion constituents presently used and the rates used by industry are summarized below:
Oil: Unsaturated fatty acids, including oleic acid ethyl ester are most effective, probably because of their molecular structure and bonding, which enable them to penetrate into the cuticle and alter the arrangement of the wax components. Saturated fatty acids are less effective.
Alkali salt: Potassium (K+) is one of the most effective cations, presumably because of its smaller ion radius when hydrated (combined with water), as compared to that of sodium, lithium, or calcium. Carbonate (CO3=) is more effective than other anions, including chloride and hydroxide.
Rates: The original fruit dipping solutions consisted of 2.5 percent K2CO3 (by weight) and 2 percent ethyl oleate (by volume); now commercial recommendations have been reduced to 2.0 percent K2CO3 and 1.6 percent ethyl oleate. If spraying onto rack-dried fruit, as is the practice in Australia, lower-strength formulations of 1.25 percent K2CO3 and 1.0 percent ethyl oleate are used. For DOV, 0.6 percent K2CO3 and 0.5 percent ethyl oleate have proven satisfactory in Australia.
Drying Emulsion Use and Research in California
Interest in the use of drying emulsions in California during the 1970s to mid-1980s was prompted by the potential of DOV to help mechanize harvest and to minimize losses from rain during tray drying. Research by California State University, Fresno and USDA Agriculture Research Service personnel during the period largely confirmed experience reported from Australia with regard to emulsion formulation. The Australian DOV practice of cutting canes and spraying fruit with the emulsion was evaluated, and some commercial lots were produced. However, vine yields went down over time as a result of cane cutting and damage to the remaining canopy from the application of emulsion. Also, the market for the treated raisins, which had different appearance and flavor characteristics, was very limited and not economically favorable.
The spray-on-tray (SOT) treatment with the emulsion was also developed by CSU Fresno researchers as a means to shorten drying time and thus rain risk. Once the grapes were spread on the trays they were sprayed with emulsion from a tractor straddling an elevated terrace. Recommended rates were 2 percent ethyl oleate (by volume) and 2 percent K2CO3 (by weight). Drying rates were increased 30 to 50 percent with this practice, provided the application was made within 2 days of harvest. There was little or no advantage in a second spray after tray turning. By 1984, 7,745 tons of raisins were produced with this method. It was largely abandoned by 1988 due to the added production costs and absence of premium prices for SOT raisins.
Today drying emulsions have limited use in California, but they may be useful in the future. They may have a place in California DOV practice, especially at the low application rates. Also, cold dips (ethyl oleate + K2CO3) can be used in tunnel dehydration rather than the hot, caustic soda dip in current use. While the cold dip may take several more hours to tunnel dry, it produces a less sticky and more free-flowing product than the hot dips.
Ref: L. Peter Christensen and William L. Peacock
